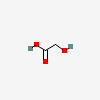
Glycolic Acid
- C2H4O3
- HOCH2COOH
- glycolic acid
- 2-Hydroxyacetic acid
- hydroxyacetic acid
- 79-14-1
- Hydroxyethanoic acid
- Create:2004-09-16
- Modify:2025-01-18
 Ammonium glycolate (is active moiety of); Glycolic acid; salicylic acid (component of); Glycerin; glycolic acid (component of) ... View More ...
Ammonium glycolate (is active moiety of); Glycolic acid; salicylic acid (component of); Glycerin; glycolic acid (component of) ... View More ...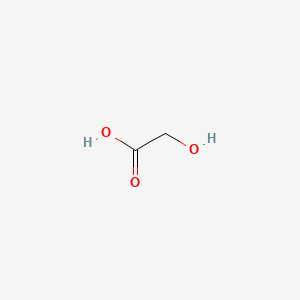
C2H4O3
HOCH2COOH
1932-50-9 (mono-potassium salt)
25904-89-6 (unspecified potassium salt)
2836-32-0 (mono-hydrochloride salt)
35249-89-9 (mono-ammonium salt)
39663-84-8 (mono-lithium salt)
- glycolate
- glycolic acid
- glycolic acid, 1-(14)C-labeled
- glycolic acid, 2-(14)C-labeled
- glycolic acid, calcium salt
- glycolic acid, monoammonium salt
- glycolic acid, monolithium salt
- glycolic acid, monopotassium salt
- glycolic acid, monosodium salt
- glycolic acid, potassium salt
- hydroxyacetic acid
- potassium glycolate
- glycolic acid
- 2-Hydroxyacetic acid
- hydroxyacetic acid
- 79-14-1
- Hydroxyethanoic acid
- Glycollic acid
- Acetic acid, hydroxy-
- glycolate
- Polyglycolide
- Acetic acid, 2-hydroxy-
- Caswell No. 470
- Kyselina glykolova
- Kyselina hydroxyoctova
- 2-Hydroxyethanoic acid
- HOCH2COOH
- alpha-Hydroxyacetic acid
- EPA Pesticide Chemical Code 000101
- HSDB 5227
- NSC 166
- Kyselina glykolova [Czech]
- AI3-15362
- MFCD00004312
- Kyselina hydroxyoctova [Czech]
- Glycocide
- GlyPure
- BRN 1209322
- NSC-166
- EINECS 201-180-5
- UNII-0WT12SX38S
- GlyPure 70
- 0WT12SX38S
- CCRIS 9474
- DTXSID0025363
- CHEBI:17497
- .alpha.-Hydroxyacetic acid
- GLYCOLLATE
- DTXCID105363
- NSC166
- EC 201-180-5
- 4-03-00-00571 (Beilstein Handbook Reference)
- GOA
- GLYCOLIC ACID (MART.)
- GLYCOLIC ACID [MART.]
- C2H3O3-
- Glycolate Standard: C2H3O3- @ 1000 microg/mL in H2O
- glycolicacid
- C2H4O3
- Hydroxyethanoate
- a-Hydroxyacetate
- OceanBlu Barrier
- OceanBlu Pre-Post
- hydroxy-acetic acid
- 2-Hydroxyaceticacid
- 3-((3S,8R,9S,10R,13S,14S)-3-ethoxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)pyridine
- alpha-Hydroxyacetate
- (R)-hydroxy ester
- a-Hydroxyacetic acid
- 2-hydroxy acetic acid
- 2-hydroxy-acetic acid
- 2-hydroxyl ethanoic acid
- Glycolic acid (Standard)
- HO-CH2-COOH
- Glycolic acid 100 microg/mL in Acetonitrile
- Hydroxyacetic acid solution
- bmse000245
- WLN: QV1Q
- GLYCOLIC ACID [MI]
- Glycolic acid (7CI,8CI)
- GLYCOLIC ACID [VANDF]
- Glycolic acid, p.a., 98%
- pari 30% Glycolic Acid Peel
- pari 70% Glycolic Acid Peel
- Acetic acid, hydroxy- (9CI)
- CHEMBL252557
- GLYCOLIC ACID [WHO-DD]
- CHEBI:17375
- Glycolic Acid, Crystal, Reagent
- HYDROXYACETIC ACID [HSDB]
- CHEBI:231641
- BCP28762
- Glycolic acid, >=97.0% (T)
- HY-W015967R
- STR00936
- (2S)-2-hydroxy monocarboxylic acid
- Tox21_301298
- (2S)-2-hydroxy monocarboxylic acids
- s6272
- STL197955
- AKOS000118921
- Glycolic acid, ReagentPlus(R), 99%
- CS-W016683
- DB03085
- HY-W015967
- SB83760
- CAS-79-14-1
- USEPA/OPP Pesticide Code: 000101
- NCGC00160612-01
- NCGC00160612-02
- NCGC00257533-01
- DA-73802
- 1ST000963
- Glycolic acid solution, 70 wt. % in H2O
- G0110
- G0196
- NS00009686
- EN300-19242
- Glycolic acid, SAJ special grade, >=98.0%
- C00160
- C03547
- D78078
- Glycolic acid, Vetec(TM) reagent grade, 98%
- HYDROXYACETIC ACID; HYDROXYETHANOIC ACID
- Glycolic acid, BioXtra, >=98.0% (titration)
- Q409373
- J-509661
- F2191-0224
- Hydroxyacetic acid; Hydroxyethanoic acid; Glycollic acid
- Z104473274
- 287EB351-FF9F-4A67-B4B9-D626406C9B13
- Glycolic acid, certified reference material, TraceCERT(R)
- InChI=1/C2H4O3/c3-1-2(4)5/h3H,1H2,(H,4,5
- VILLAGE 11 FACTORY RELAX DAY AHA EXFOLIATING BODY
- Glycolic acid, anhydrous, free-flowing, Redi-Dri(TM), ReagentPlus(R), 99%
- Glycolic Acid, Pharmaceutical Secondary Standard; Certified Reference Material
- O7Z
31.0 99.99
32.0 33.88
29.0 26.11
45.0 14.09
30.0 5.59
31.0 99.99
32.0 31.97
29.0 27.06
49.0 14.08
45.0 12.50
76.9 97.66
48.9 2.34
76.9 93.15
48.9 6.85
74.9 999
73 6
46.7 3
44.9 3
31.3 2
75.1 999
47.1 62
45.2 50
72.9 48
31.3 45
31 999
32 339
29 261
45 141
30 56
Ammonium glycolate (is active moiety of)
- Glycolic acid; salicylic acid (component of)
- Glycerin; glycolic acid (component of)
- Glycolic acid; salicylic acid; sulfur (component of)
- Benzoyl Peroxide; GLYCOLIC ACID (component of)
- Alcohol; ammonia; ascorbyl glucoside; ethylhexylglycerin; glycolic acid; hexylresorcinol; hydroxyethyl cellulose, unspecified; isopropyl alcohol; kojic acid; lactic acid; phenoxyethanol; propylene glycol; salicylic acid; water (component of)
- Polyglycolide (annotation moved to)
- Bladder
- Epidermis
- Fibroblasts
- Liver
- Mitochondria
- Peroxisome
Acid and Alkali Cleaning of Metals [Category: Clean]
Electroplating [Category: Plate]
Petroleum Production and Refining [Category: Industry]
Soldering [Category: Heat or Machine]
Working with Glues and Adhesives [Category: Other]
Leather Tanning and Processing [Category: Industry]
Fur Dressing and Dyeing [Category: Industry]
Textiles (Printing, Dyeing, or Finishing) [Category: Industry]
- Processing aids, not otherwise listed
- Cleaning agent
- Dye
- Intermediates
- Not Known or Reasonably Ascertainable
- Intermediate
- Oxidizing/reducing agents
- Solvents (for cleaning or degreasing)
- Other (specify)
- Dye
- Cleaning agent
- Processing aids, not otherwise listed
- Plating agents and surface treating agents
- Agricultural chemicals (non-pesticidal)
- Laboratory chemicals
- Not Known or Reasonably Ascertainable
Information on 117 consumer products that contain Hydroxyacetic acid in the following categories is provided:
• Auto Products
• Commercial / Institutional
• Home Maintenance
• Inside the Home
• Personal Care
• Pet Care
2019: 20,000,000 lb - <100,000,000 lb
2018: 20,000,000 lb - <100,000,000 lb
2017: 20,000,000 lb - <100,000,000 lb
2016: 20,000,000 lb - <100,000,000 lb
- All Other Chemical Product and Preparation Manufacturing
- Not Known or Reasonably Ascertainable
- Plastics Material and Resin Manufacturing
- Soap, Cleaning Compound, and Toilet Preparation Manufacturing
- Utilities
- Paper Manufacturing
- All Other Basic Organic Chemical Manufacturing
- Petroleum Refineries
- Petroleum Lubricating Oil and Grease Manufacturing
- Food, beverage, and tobacco product manufacturing
- Wholesale and Retail Trade


H302 (82.5%): Harmful if swallowed [Warning Acute toxicity, oral]
H314 (99.7%): Causes severe skin burns and eye damage [Danger Skin corrosion/irritation]
H318 (28.1%): Causes serious eye damage [Danger Serious eye damage/eye irritation]
H332 (30.2%): Harmful if inhaled [Warning Acute toxicity, inhalation]
P260, P261, P264, P264+P265, P270, P271, P280, P301+P317, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P317, P321, P330, P363, P405, and P501
(The corresponding statement to each P-code can be found at the GHS Classification page.)
Aggregated GHS information provided per 3293 reports by companies from 34 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies.
Reported as not meeting GHS hazard criteria per 10 of 3293 reports by companies. For more detailed information, please visit ECHA C&L website.
There are 33 notifications provided by 3283 of 3293 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
Acute Tox. 4 (82.5%)
Skin Corr. 1A (99.7%)
Eye Dam. 1 (28.1%)
Acute Tox. 4 (30.2%)
Acute toxicity (Oral) - Category 4
Acute toxicity (Inhalation: Dusts and mists) - Category 4
Skin corrosion/irritation - Category 1B
Serious eye damage/eye irritation - Category 1
Reproductive toxicity - Category 2
Specific target organ toxicity - Single exposure - Category 1 (respiratory system)
Specific target organ toxicity - Repeated exposure - Category 2 (liver, thymus)
Hazardous to the aquatic environment (Acute) - Category 3
Chemical: Acetic acid, hydroxy-
Specific Information Requirement: Obligations to provide information apply. You must tell us within 28 days if the circumstances of your importation or manufacture (introduction) are different to those in our assessment.
 Chemical Not Tested in Species/Sex
Chemical Not Tested in Species/Sex Chemical Not Tested in Species/Sex
Chemical Not Tested in Species/Sex No Evidence
No Evidence No Evidence
No Evidence◉ Summary of Use during Lactation
No information is available on the clinical use of glycolic acid (hydroxyacetic acid) on the skin during breastfeeding. Because it is unlikely to be appreciably absorbed or appear in breastmilk, it is considered safe to use during breastfeeding. Avoid application to areas of the body that might come in direct contact with the infant's skin or where the drug might be ingested by the infant via licking.
◉ Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Nephrotoxin - The chemical is potentially toxic to the kidneys in the occupational setting.
Dermatotoxin - Skin burns.
Silke Matysik, Caroline Ivanne Le Roy, Gerhard Liebisch, Sandrine Paule Claus. Metabolomics of fecal samples: A practical consideration. Trends in Food Science & Technology. Vol. 57, Part B, Nov. 2016, p.244-255: http://www.sciencedirect.com/science/article/pii/S0924224416301984
PubMed: 8134166, 6774165, 11999977, 8981317
MetaGene: Metabolic & Genetic Information Center (MIC: http://www.metagene.de)
PubMed: 26078636, 20549362, 24182348, 6616883, 16972175
MetaGene: Metabolic & Genetic Information Center (MIC: http://www.metagene.de)
PubMed: 1458609
Primary Hyperoxaluria Type 1. 2002 Jun 19 [Updated 2014 Jul 17]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1283/
Patents are available for this chemical structure:
https://patentscope.wipo.int/search/en/result.jsf?inchikey=AEMRFAOFKBGASW-UHFFFAOYSA-N
- Australian Industrial Chemicals Introduction Scheme (AICIS)Acetic acid, hydroxy-https://services.industrialchemicals.gov.au/search-assessments/Acetic acid, hydroxy-https://services.industrialchemicals.gov.au/search-inventory/
- CAS Common ChemistryLICENSEThe data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc/4.0/Glycolic acid polymerhttps://commonchemistry.cas.org/detail?cas_rn=26124-68-5
- ChemIDplusAcetic acid, 2-hydroxy-, homopolymerhttps://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0026124685ChemIDplus Chemical Information Classificationhttps://pubchem.ncbi.nlm.nih.gov/source/ChemIDplus
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useGlycolic acidhttps://www.drugbank.ca/drugs/DB03085
- DTP/NCILICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- EPA Chemical Data Reporting (CDR)LICENSEThe U.S. Government retains a nonexclusive, royalty-free license to publish or reproduce these documents, or allow others to do so, for U.S. Government purposes. These documents may be freely distributed and used for non-commercial, scientific and educational purposes.https://www.epa.gov/web-policies-and-procedures/epa-disclaimers#copyrightAcetic acid, 2-hydroxy-https://www.epa.gov/chemical-data-reporting
- EPA Chemicals under the TSCAAcetic acid, 2-hydroxy-https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- EPA DSSToxGlycolic acidhttps://comptox.epa.gov/dashboard/DTXSID0025363CompTox Chemicals Dashboard Chemical Listshttps://comptox.epa.gov/dashboard/chemical-lists/
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeGlycollic acidhttps://chem.echa.europa.eu/100.001.073Glycollic acid (EC: 201-180-5)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/22074
- FDA Global Substance Registration System (GSRS)LICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Hazardous Substances Data Bank (HSDB)HYDROXYACETIC ACIDhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/5227
- Human Metabolome Database (HMDB)LICENSEHMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications.http://www.hmdb.ca/citingGlycolic acidhttp://www.hmdb.ca/metabolites/HMDB0000115HMDB0000115_cms_29929https://hmdb.ca/metabolites/HMDB0000115#spectra
- ILO-WHO International Chemical Safety Cards (ICSCs)HYDROXYACETIC ACIDhttps://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=1537
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- ChEBI
- E. coli Metabolome Database (ECMDB)
- LOTUS - the natural products occurrence databaseLICENSEThe code for LOTUS is released under the GNU General Public License v3.0.https://lotus.nprod.net/Glycolic acidhttps://www.wikidata.org/wiki/Q409373LOTUS Treehttps://lotus.naturalproducts.net/
- Open TargetsLICENSEDatasets generated by the Open Targets Platform are freely available for download.https://platform-docs.opentargets.org/licenceGLYCOLIC ACIDhttps://platform.opentargets.org/drug/CHEMBL252557
- Toxin and Toxin Target Database (T3DB)LICENSET3DB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (T3DB) and the original publication.http://www.t3db.ca/downloadsGlycolic acidhttp://www.t3db.ca/toxins/T3D3660
- ChEMBLLICENSEAccess to the web interface of ChEMBL is made under the EBI's Terms of Use (http://www.ebi.ac.uk/Information/termsofuse.html). The ChEMBL data is made available on a Creative Commons Attribution-Share Alike 3.0 Unported License (http://creativecommons.org/licenses/by-sa/3.0/).http://www.ebi.ac.uk/Information/termsofuse.htmlChEMBL Protein Target Treehttps://www.ebi.ac.uk/chembl/g/#browse/targets
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jspglycolic acidhttps://ctdbase.org/detail.go?type=chem&acc=C031149
- Therapeutic Target Database (TTD)Hydroxyacetic Acidhttps://idrblab.net/ttd/data/drug/details/D01HNP
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Hydroxyacetic acidhttps://www.whatsinproducts.com/chemicals/view/1/12/000079-14-1Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- Cosmetic Ingredient Review (CIR)
- EPA Chemical and Products Database (CPDat)EPA CPDat Classificationhttps://www.epa.gov/chemical-research/chemical-and-products-database-cpdat
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutGlycolic acidhttps://haz-map.com/Agents/1410
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/Glycolic acidNORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- Crystallography Open Database (COD)LICENSEAll data in the COD and the database itself are dedicated to the public domain and licensed under the CC0 License. Users of the data should acknowledge the original authors of the structural data.https://creativecommons.org/publicdomain/zero/1.0/
- DailyMed
- IUPAC Digitized pKa Datasetacetic acid, hydroxy-https://github.com/IUPAC/Dissociation-Constants
- Drugs and Lactation Database (LactMed)Glycolic Acidhttps://www.ncbi.nlm.nih.gov/books/n/lactmed/LM1321/
- ECI Group, LCSB, University of Luxembourgglycolic acid
- Natural Product Activity and Species Source (NPASS)
- NITE-CMCHydroxyacetic acid - FY2012 (New/original classication)https://www.chem-info.nite.go.jp/chem/english/ghs/12-mhlw-0051e.html
- NMRShiftDB
- MassBank Europe
- MassBank of North America (MoNA)LICENSEThe content of the MoNA database is licensed under CC BY 4.0.https://mona.fiehnlab.ucdavis.edu/documentation/license
- NIST Mass Spectrometry Data CenterLICENSEData covered by the Standard Reference Data Act of 1968 as amended.https://www.nist.gov/srd/public-lawAcetic acid, hydroxy-http://www.nist.gov/srd/nist1a.cfm
- SpectraBaseHYDROXYACETIC ACIDhttps://spectrabase.com/spectrum/IX11jj8D0nlAcetic acid, hydroxy-https://spectrabase.com/spectrum/9VwEjYJnL9glycolic acidhttps://spectrabase.com/spectrum/LQCVS8VBcXiGLYCOLIC ACIDhttps://spectrabase.com/spectrum/LVmGOSteDouGlycolic acidhttps://spectrabase.com/spectrum/KAJvIRzYP4mGlycolic acidhttps://spectrabase.com/spectrum/HlbKOUpyKCZGlycolic acidhttps://spectrabase.com/spectrum/AT7gUsEIcblAcetic acid, hydroxy-https://spectrabase.com/spectrum/JhV4OZjPBS1Acetic acid, hydroxy-https://spectrabase.com/spectrum/DQLi2BYJKQoGlycolic acidhttps://spectrabase.com/spectrum/B4PWoH2DfXHGlycolic acidhttps://spectrabase.com/spectrum/9lxh8wDJAj5
- Japan Chemical Substance Dictionary (Nikkaji)
- KEGGLICENSEAcademic users may freely use the KEGG website. Non-academic use of KEGG generally requires a commercial licensehttps://www.kegg.jp/kegg/legal.html
- MarkerDBLICENSEThis work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.https://markerdb.ca/Glycolic acidhttps://markerdb.ca/chemicals/70
- Metabolomics Workbench
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Nature Chemical Biology
- Nature Chemistry
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuseNCI Thesaurushttps://ncit.nci.nih.gov
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlglycolic acidhttps://rxnav.nlm.nih.gov/id/rxnorm/587318
- NTP Technical ReportsGlycolic Acid (Photocarcinogenesis Study)https://ntp.niehs.nih.gov/data/tr
- Protein Data Bank in Europe (PDBe)
- RCSB Protein Data Bank (RCSB PDB)LICENSEData files contained in the PDB archive (ftp://ftp.wwpdb.org) are free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use. Users of the data should attribute the original authors of that structural data.https://www.rcsb.org/pages/policies
- Rhea - Annotated Reactions DatabaseLICENSERhea has chosen to apply the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). This means that you are free to copy, distribute, display and make commercial use of the database in all legislations, provided you credit (cite) Rhea.https://www.rhea-db.org/help/license-disclaimer
- Springer Nature
- Thieme ChemistryLICENSEThe Thieme Chemistry contribution within PubChem is provided under a CC-BY-NC-ND 4.0 license, unless otherwise stated.https://creativecommons.org/licenses/by-nc-nd/4.0/
- Wikidatahydroxyacetic acidhttps://www.wikidata.org/wiki/Q409373
- WikipediaPolyglycolidehttps://en.wikipedia.org/wiki/PolyglycolideEthyl cinnamatehttps://en.wikipedia.org/wiki/Ethyl_cinnamateGlycolic acidhttps://en.wikipedia.org/wiki/Glycolic_acid
- Wiley
- PubChem
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlglycolic acidhttps://www.ncbi.nlm.nih.gov/mesh/67031149Keratolytic Agentshttps://www.ncbi.nlm.nih.gov/mesh/68007641
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- EPA Substance Registry ServicesEPA SRS List Classificationhttps://sor.epa.gov/sor_internet/registry/substreg/LandingPage.do
- MolGenieMolGenie Organic Chemistry Ontologyhttps://github.com/MolGenie/ontology/
- PATENTSCOPE (WIPO)SID 403029864https://pubchem.ncbi.nlm.nih.gov/substance/403029864
- NCBI